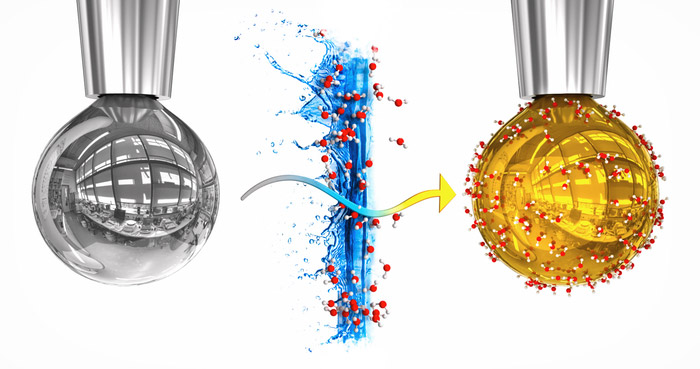
Miracle change: For the first time researchers have provided water with metallic properties – without much pressure and at room temperature. This is achieved by a Nifty trick: they allow water vapor to condense in a drop of liquid alkali metals in a vacuum. The migration of electrons from the metal gave a thin layer of water the conductivity and appearance of a metal for some time: the drop acquired a golden luster.
Metals have good conductivity due to them External electrons The nuclear rod can move freely. This is different in distilled water: because the outer electrons of the atoms are firmly bonded, and pure water is an almost perfect insulator. To conduct it and thus metallize, one must press the water molecules strongly. The high pressure then unites the orbits of the outer electrons very closely. Due to the increased electron density, some electrons become mobile, thus allowing the flow of current.
The problem, however, is explained by Philip Mason: “For pure water, the pressure required for this is estimated at 48 megabars – which is beyond our experimental possibilities today and probably occurs only at the center of large planets or stars.” Czech Academy of Sciences Brock and his colleagues. In fact, there is some evidence that hydrogen, helium and even water may be present Inside Thursday May be metal.
Alkali metals and water – a soft compound
As researchers now prove in one experiment, there is another way. The starting point for this is the idea that the electron density of water can be increased by stimulants – by injecting extra electrons from another substance. It has been known from previous experiments that alkali metals are in principle electron donors.
As for the mixture of alkali metals and water, there is one important obstacle – as most of you know from school: “If you throw a lump of sodium into the water, the metal will not get water, but an immediate and violent explosion,” explains Mason’s colleague Pavel Jangwirth. “To avoid this violent and negative reaction, we did everything else: we added water to the metal.” The eruption did not occur because the amount of water was so small.
The drop will turn to shiny gold
In particular, the test works like this: a small drop of sodium-potassium solution emerges from the particles in the high vacuum sample chamber. The silver drop grows about ten seconds before falling away from the tip. When the drop forms, the researchers inject water vapor into the chamber. Some water molecules are placed on the surface of water droplets and form a layer of water that is thickened by about 80 molecular layers per second.
Interesting thing about it: once the first water molecules are deposited on the metal droplet, its color changes. “The silver sodium-potassium drop has a golden sheen, which is very interesting,” said Robert Seidel, co-editor of the Helmholtz Center for Materials and Energy in Berlin. The metallic gold luster is retained for about five seconds before the drop color finally changes from bronze and purple to white.
Free electrons in the water layer
This color change represents a decisive change in the electrochemical behavior of the water layer. As confirmed by spectroscopic analyzes, non-conductive water is conductive at the time of exposure – it acquires metallic properties. “Our observations are that the golden color and its characteristic luster indicate the metallicity of the water surface layer, writes Mason and his colleagues.
This can be measured as the researchers explain that a fluctuating charge called plasmon and a conduction band with a width of about 1.1 electron volts are formed. Both now indicate the presence of freely moving electrons in water. They come from alkaline metals and are transferred to the water layer after the water molecules have accumulated. According to measurements, the density of these extra electrons during the gold phase is five trillion electrons per cubic centimeter.
This is how water change works.Under Thunderf00t
Metal without high pressure
This test proves what was previously considered impossible: water can metallize even without extreme pressure. In the drop test, this condition, abnormal for pure water, lasted for a few seconds, the researchers said. However, the chemical reaction of water with alkaline metals leads to the formation of white alkaline hydroxide.
“Our study not only shows that metallic water can actually be produced on Earth, but also characterizes the spectroscopic properties associated with its beautiful golden metallic luster,” says Seidel. This will make it possible to research the properties and behaviors of metallic water even without high pressure in the future. (Nature, 2021; doi: 10.1038 / s41586-021-03646-5)
Source: Helmholtz Center for Materials and Energy Berlin, Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences

“Avid writer. Subtly charming alcohol fanatic. Total twitter junkie. Coffee enthusiast. Proud gamer. Web aficionado. Music advocate. Zombie lover. Reader.”










More Stories
What Does the Future of Gaming Look Like?
Throne and Liberty – First Impression Overview
Ethereum Use Cases