Physicists led by Roland Wester of the University of Innsbruck meticulously observed the rivalry between two important reaction mechanisms in organic chemistry in the laboratory. The detailed investigation of the reaction dynamics of a reaction complex with nine atoms is unique so far. In doing so, scientists are advancing to a certain extent, implementing applications in many areas of chemistry.
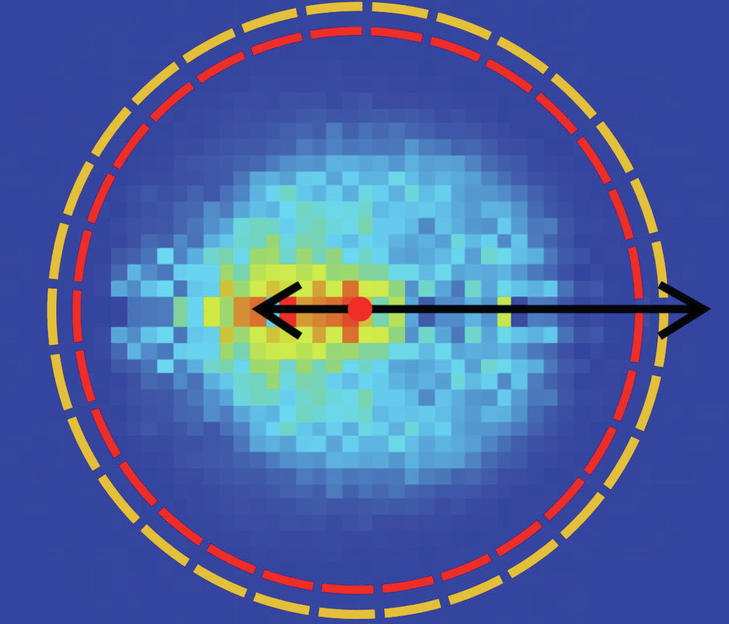
Through laboratory experiments, Roland Wester, a two-time ERC Award winner from the Institute of Ian Physics and Applied Physics at the University of Innsbruck, seeks to look at chemical reactions and better understand their dynamics. The reactions of small molecules are well understood today. Once more than four atoms are involved, it is difficult to describe in detail the course of the reaction for both theory and experiment. Roland Wester and his team have developed a unique experiment in which molecules can be observed by reacting with ions. For the first time, scientists have succeeded in accurately describing nuclear dynamics known as the nucleophilic alternative reaction. A few years ago, a research team studied the competition between two chemical reactions in organic compounds. In a vacuum chamber, the researchers collided these molecules with charged particles from a chemical group of halogens such as fluorine, iodine and chlorine. For the first time, direct observation showed that the removal reaction with large molecules gets the upper hand and the alternative reaction disappears at some point.
High dimensional reaction dynamics
It is unknown at this time what he will do after leaving the post. To investigate this, more theoretical work was needed, which was carried out in recent years by researchers led by Koper Soke at the Sazet University in Hungary. They calculated the so-called Born-Oppenheimer potential surface, with which the chemical reaction of a molecule can be described in detail with the eight-atom halogen ion. It is noteworthy that the reaction studied takes place in 21 dimensions. Provides information on how individual atoms move in this high dimensional space during a theoretically determined potential surface chemical reaction. From this, Innbrook scientists working with Eduardo Karascoza, Jennifer Meyer and Roland Wester were able to make an accurate prediction of the spatial direction in which the reactant material would fly in their experiment. Because they measure the angle and the speed at which the ions hit a detector. “Our data shows that we measured exactly what the theory calculated without knowing the experimental data,” says Wester happily. “With so many atoms and so many dimensions, this has not yet been achieved.”
Scientists have thus described chemical reactions in detail, in which two different reaction mechanisms take place theoretically and experimentally. Through this work, now published in the journal Nature Chemistry, Innsbruck physicists are coming to a region where the number of atoms is subject to extensive investigation of the reaction mechanics that drive applications in many areas of complex chemical reactions.

“Avid writer. Subtly charming alcohol fanatic. Total twitter junkie. Coffee enthusiast. Proud gamer. Web aficionado. Music advocate. Zombie lover. Reader.”










More Stories
Acrylic Nails for the Modern Professional: Balancing Style and Practicality
The Majestic Journey of the African Spurred Tortoise: A Guide to Care and Habitat
Choosing Between a Russian and a Greek Tortoise: What You Need to Know